近日,我校理学院陈浩教授领衔的“先进材料与绿色催化”团队项勇刚教授课题组在模拟人工光合作用研究方面取得最新进展,设计出系列新型有机共轭聚合物光催化材料,大幅提升了光生电荷的分离效率,实现了太阳能向清洁化学能源——氢能的高效转化过程。
随着全球能源短缺和环境污染问题日益严重,开发利用清洁的可再生能源迫在眉睫。氢气是未来人类社会可持续发展的理想能源,利用太阳光驱动的光催化制氢技术为解决能源与环境问题提供了新的契机。受自然界光合作用过程中的光合系统I(PSI)对太阳光吸收以及影响化学反应过程的电荷分离机制启发,项勇刚教授课题组开展了具有宽光谱吸收及优异电荷分离的电子给体(D)-电子受体(A)型共轭聚合物仿生光催化体系的开发研究。在前期工作中,课题组研究人员发现通过在电子受体单元中引入强电负性氟原子能够有效构建具有良好电荷分离能力的弱D-强A型共轭聚合物,其光催化制氢性能得到极大提升,然而进一步有效调控材料电子结构提高其光催化效率仍然面临巨大挑战。
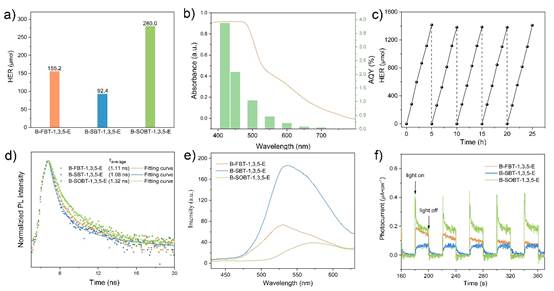
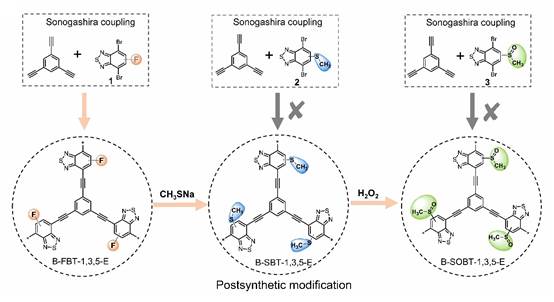
在本研究中,基于理论计算模拟首次发现具有强电负性亚磺酰基(CH3SO-)相对于氟(F)可进一步促进苯并噻二唑基共轭聚合物电子传输效率。但是传统的聚合方式无法直接制备获得亚磺酰基(CH3SO-)取代的共轭聚合物B-SOBT-1,3,5-E。基于此,课题组研究人员创新性采用了共轭聚合物连续后修饰(PSM)的合成策略,首先将F取代B-FBT-1,3,5-E通过芳香亲核取代反应(SNAr)转变为具有给电子特性的甲硫基(CH3S-)取代B-SBT-1,3,5-E,进一步采用氧化反应将甲硫基转化成亚磺酰基。通过固体核磁、红外及XPS等表征手段证实了这种连续后修饰策略均具有高效的化学转化效率。通过光催化制氢实验研究发现,B-FBT-1,3,5-E,B-SBT-1,3,5-E和B-SOBT-1,3,5-E的HER分别为155.2 μmol h-1,92.4 μmol h-1和280.0 μmol h-1,符合理论计算模拟对共轭聚合物电子传输效率的预测。进一步,通过时间分辨光致发光谱及光电流等光电性能测试表明,共轭聚合物组成单元的电子结构调控对其电荷分离及界面反应具有决定性作用。此外,研究还将该策略成功的扩展到其他三个氟取代苯并噻二唑基共轭聚合物体系中,证实了亚磺酰基引入对实现光催化制氢效率的提高具有普适性。上述研究成果为设计合成具有良好光催化产氢活性的有机聚合物并将其应用于新型人工光合作用体系,实现太阳能向化学能的高效转化提供了新的思路。
论文链接:https://pubs.rsc.org/en/Content/ArticleLanding/2021/TA/D1TA01074C#!divAbstract
英文摘要:Donor/Acceptor (D-A) conjugated polymers recently represent a promising platform for photocatalytic H2 evolution, and a variety of methods have been developed to expand the library of conjugated polymer based candidates. However, strategies to facilely modulate the electronic structures is still a challenge. Herein, we demonstrate that replacement of electron-withdrawing fluorine (F) in the F substituted benzothiadiazole (BT) based conjugated polymers with more electron-withdrawing group methylsulfinyl (CH3SO-) significantly increases the photocatalytic H2 evolution rate (HER). To overcome the failure of direct polymerization, a two consecutive postsynthetic modifications (PSM) approach was innovatively adopted. F substituted B-FBT-1,3,5-E was first converted into the electro-donating group methylthio (CH3S-) modified B-SBT-1,3,5-E via SNAr reaction, and subsequent oxidization afforded the CH3SO- substituted B-SOBT-1,3,5-E. Photocatalytic H2 evolution experiments results reveal that HER of B-FBT-1,3,5-E, B-SBT-1,3,5-E and B-SOBT-1,3,5-E is 155.2 μmol h-1, 92.4 μmol h-1 and 280.0 μmol h-1, respectively, and the best performance of B-SOBT-1,3,5-E is attributed to the more electron-withdrawing and hydrophilic property of CH3SO-. Such protocol can also be extended to three more series of BT based conjugated polymers with benzene replaced, highlighting excellent property of sulfinyl group for designing polymer based photocatalysts toward solar-to-chemical energy conversion in the near future.
